Erectile dysfunction: Topical gel gets FDA nod for over-the-counter marketing
Futura Medical has developed Eroxon, a topical gel for erectile dysfunction, which has been authorized for over-the-counter marketing in the US. The product, already available in Belgium and the UK, is the first of its kind available without a prescription.
Futura Medical has developed Eroxon, a topical gel for erectile dysfunction, which has been authorized for over-the-counter marketing in the US. The product, already available in Belgium and the UK, is the first of its kind available without a prescription.

Proposition - PharmXpert Academy

FDA grants OTC marketing clearance to topical gel for erectile

FDA approves Eroxon over-the-counter gel to treat erectile dysfunction

FDA approves Eroxon as a treatment for erectile dysfunction - The

FDA clears erectile dysfunction gel for over the counter

The US FDA OTC Approval of Topical Gel for Erectile Dysfunction

Imiquimod 3.75% Cream- 7.5g Pump
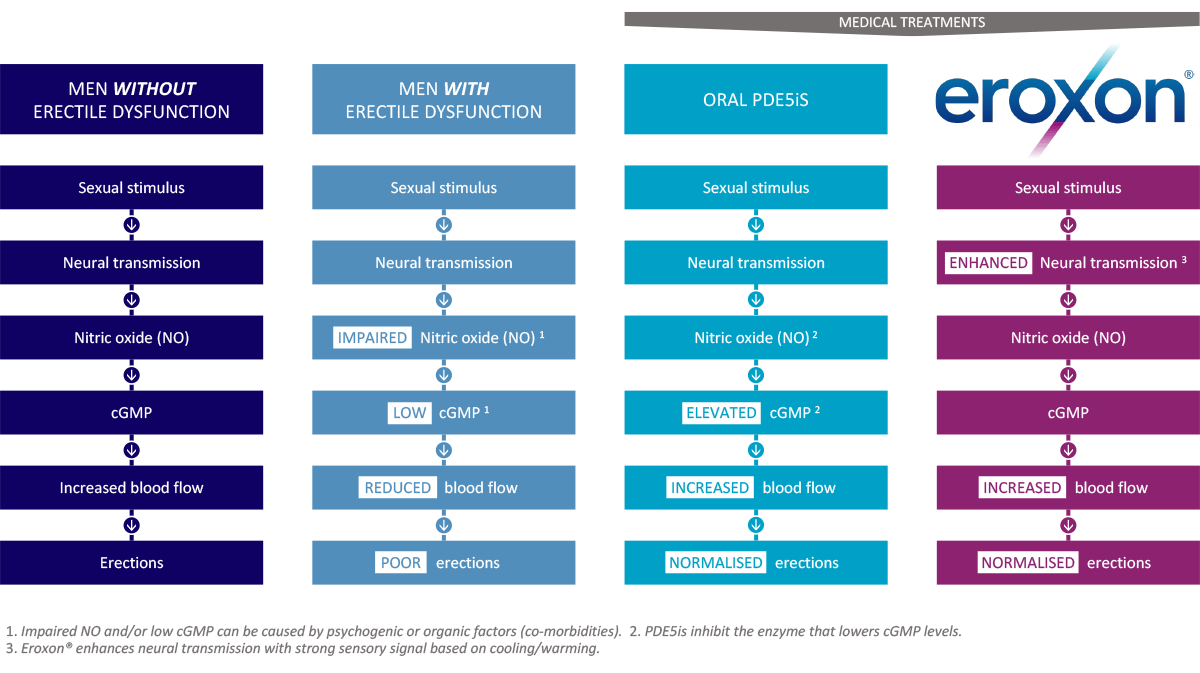
MED3000 CLINICAL PROGRAMME - Futura Medical
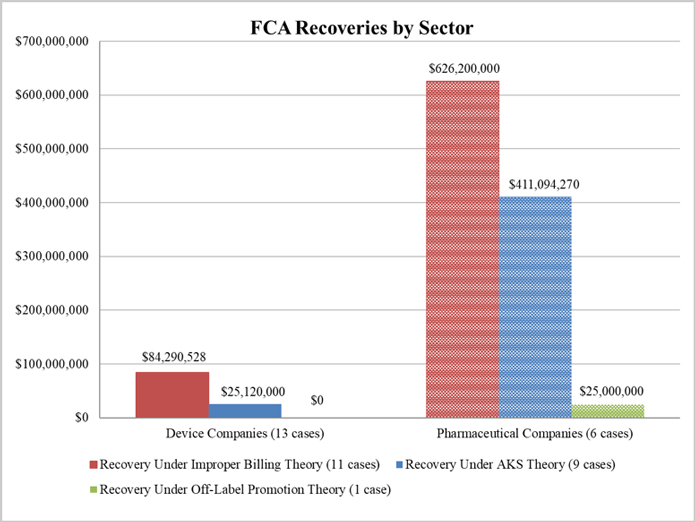
2018 Year-End FDA and Health Care Compliance and Enforcement

CBD Oil for Pain: Does It Work, Arthritis, Cancer & More

10 Best AI Email Marketing Tools & Platforms in 2024!

There's Now an OTC Gel for Erectile Dysfunction

FDA greenlights first OTC topical gel for erectile dysfunction